Until recently, most soil testing procedures assessed soil fertility, in order to guide appropriate application of nutrient-based fertilizers. Soils do a lot more than just make nutrients available, however. They hold and filter water, cycle nutrients, stabilize organic matter, create habitat for a vast array of organisms, and potentially mitigate against climate change by binding carbon within the soil. Recent years have seen an explosion of new soil health tests that measure organic matter decomposition, nutrient cycling, aggregate stability, carbon sequestration—all of which depend on the activity of soil microorganisms. This publication describes several of the most common soil health assessment methods, including total soil organic matter, active organic matter, soil respiration, aggregate stability, and the Haney soil test. We identify benefits and limitations for each method and provide suggestions and resources for conducting these analyses on your farm or ranch.

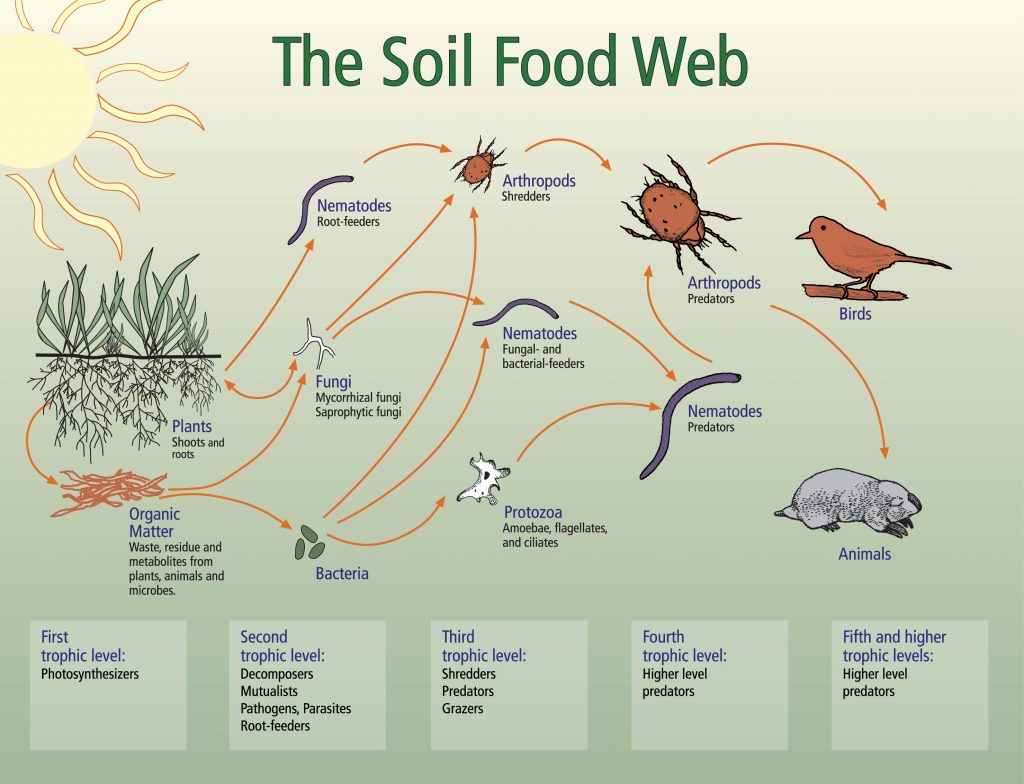
The soil food web focuses on micro and larger organisms involved in decomposition. Microorganisms provide many other services and benefits. Source: Ingham, no date.
While farmers have understood the importance of good-quality soil since the dawn of agriculture, and described it with terms like “soil tilth” and (in some cultures) “fat soil,” the concept of soil health only began attracting the attention of scientists and educators in about the 1980s. When synthetic fertilizers were developed in the early 1900s, many soil scientists focused their research on how farmers could either apply more fertilizers or apply them more effectively, to overcome the problems of soil degradation due to erosion or nutrient loss due to crop removal. As more people left the farm and moved to cities, they started blaming farmers and their use of fertilizers and manure for algae blooms on lakes they use for recreation and drinking water. At the same time, some farmers noticed that their crop yields were decreasing while the cost of fertilizers and other inputs increased. And in the 1970s and 1980s, younger soil scientists with an interest in sustainable agriculture started challenging the dogma that soil fertility management was the solution to all soil problems.
Today, soil health has become a primary interest of the USDA Natural Resources Conservation Service (NRCS), and is the focus of many research programs, as well as emerging programs that pay farmers for protecting the environment and water quality. This publication will provide an overview of factors affecting soil health and then discuss some of the most common ways of assessing it.
What is soil health? While definitions vary, NRCS has defined it as “the continued capacity of soil to function as a vital living ecosystem that sustains plants, animals, and humans” (NRCS, no date). Notice two things about this definition: First, healthy soil is defined as being alive. Second, healthy soil is capable of carrying out certain functions. The NRCS goes on to mention five of these functions:
To really understand soil health, we need to understand what it means for a soil to be alive. Besides minerals, water, and air, soil that is alive also contains countless organisms—burrowing animals, worms, insects, and microorganisms. For these organisms to thrive, they need to eat, breathe, have access to water, and be protected against poisons. More simply put, a healthy soil must have the following characteristics (Doran and Zeiss, 2000):
What does the health of soil organisms have to do with the health and production of forages and crop plants? To mention just three of the many processes at work:
 |
 |
| Mycorrhizae extend out into the soil, bringing water and nutrients to the plant. Sources: Smith, 2015; Todd, no date |
Three types of organisms are primarily responsible for soil health characteristics: decomposers, mycorrhizae, and bacteria in the rhizosphere, or root zone, of the plant.
Decomposers include shredders, burrowers, and various microorganisms that are part of the familiar soil food web (refer to graphic above). Shredders such as birds, small animals, bugs, beetles, ants, and other insects transform large pieces of organic matter into manure and small crumbs that are left behind during eating.
Burrowers such as ants and earthworms carry pieces of organic matter into the soil, providing food for microorganisms. Microorganisms such as protozoa, fungi, and bacteria transform organic materials into inorganic nutrients that can be taken up by plants. Earthworms and microorganisms also create various types of exudates that help pieces of soil stick together to form aggregates. These slimes can also increase cation exchange capacity (CEC), or the ability of soil particles to hold onto cations (positively charged molecules), such as ammonium, potassium, calcium, and magnesium, making these nutrients available for plant uptake.
Mycorrhizae are a type of fungus that has a symbiotic (mutual assistance) relationship with most plants. (Crucifers, such as radish, cabbage, and turnips, are an exception.) Mycorrhizae begin their growth within plant root cells and get carbohydrate energy from plants. In return, the hyphae, or filamentous threads, of mycorrhizae extend much farther and into tighter pores than plant roots can reach, giving the plant up to 100 times more access to water and nutrients than that provided by the roots alone.
Mycorrhizae help plants withstand droughts and take up phosphorus: a nutrient that is often difficult for plant roots to access. Mycorrhizae can also strengthen a plant’s ability to resist pest and disease infestations and help bind soil particles together into aggregates—reducing soil compaction and providing an organic coating that enhances water- and nutrient-holding capacity. Mycorrhizae depend on plant roots for their carbohydrates, and die off or become dormant if no plants are growing in the soil.
Rhizosphere bacteria live close to plant roots, often getting their nutrients from various sugars and other chemicals that ooze out of plant roots. In return, these bacteria provide many benefits. Some break down complex mineral forms of phosphorus, such as calcium phosphate, into simpler forms that are available for plant uptake. Other rhizosphere bacteria make micronutrients like iron more available, stimulate root growth, provide protection against soil salinity, or improve resistance to plant diseases. Like decomposers, rhizosphere bacteria exude slimes that can coat soil particles, facilitating the formation of soil aggregates and enhancing nutrient- and water-holding capacity.
Rhizobacteria promote plant growth, protect against pests, and reduce toxic impacts of contaminants. Source: Kumar et al., 2011
A strip of native pasture enhancing the diversity of soil microorganisms. Source: Conservation Media Library, 2016
The NRCS has identified five general principles for building soil health:
Some farming practices that follow these principles are reduced- or no-till land preparation and seeding, crop rotations, planting diverse cover crops, and using rotational or managed grazing.
No-till and reduced-till farming reduce soil disturbance while leaving organic residues on the soil surface. These practices:

Healthy soil is dark-colored due to high organic matter content, and has good tilth or aggregate stability. Source: USDA NRCS, 2012
Crop rotations enhance the diversity of plant roots in the soil, which increases:
Diverse cover crop mixtures, including legumes, forbs, and tillage radishes:
Rotational or managed grazing with adequate rest periods benefits the soil by:
Many other land management practices also promote soil health. For example, recent research in Iowa demonstrated multiple benefits from planting native prairie strips within or at the edges of fields (Moore, 2014). Silvopasture, adaptive grazing in orchards or timber stands, alley cropping, and other mixed-species practices have been shown to improve soil health.
Soil health tests measure biological, chemical, and physical properties that indicate either healthy growth of soil organisms or properties that promote this healthy growth. Most soil health tests measure one of the following five things:
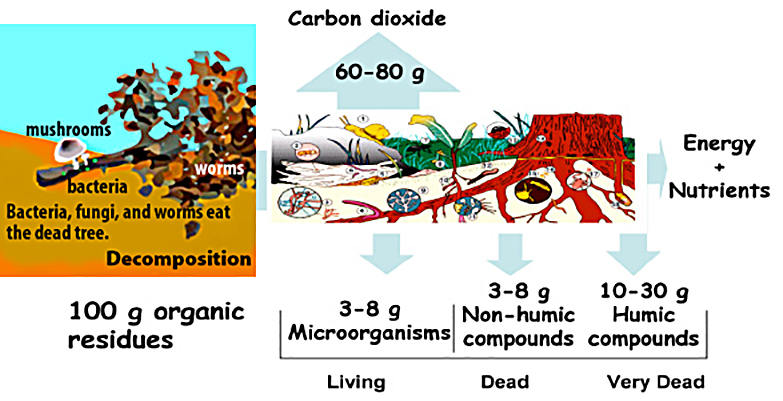
Source: Hoorman and Islam, 2010
Many of the soil health tests listed in Table 1 can be conducted in the field using a low-cost process. You may be familiar with field-based assessments like the following:
| Soil Health Characteristics | Sub-Characteristics | Soil Health Tests |
|---|---|---|
| Soil nutrients | Amount of nutrients at time of sampling | Soil nutrient test |
| Ability of the soil to not lose nutrients to leaching/runoff | Cation Exchange Capacity (CED) | |
| Availability of nutrient for plant growth | pH | |
| Soil organic matter | All forms of organic matter | Total soil organic matter |
| Living and dead organic matter | Active soil organic matter | |
| Particulate organic matter | ||
| Soil aggregates | Amount of stable aggregates | Aggregate stability |
| Organisms involved in aggregate formation | Soil protein/glomalin | |
| Diversity and growth of soil organisms | Growth (activity) of soil organisms | Soil respiration |
| Rate of decompositions by soil organisms | Nitrogen mineralizaiton | |
| Diversity and populations of various soil organisms | Phospholipid Fatty Acid (PLFA) | |
| Physical and chemical characteristics | Soil looseness or compaction | Bulk density |
| Ability of rainfall or irrigation water to easily soak into the soil | Water infiltration | |
| How gritty or sticky a soil feels | Soil texture |
More complete descriptions of field-based soil health assessments are provided on the NRCS soil health website.
Whether it is done in the field or in a commercial laboratory with sophisticated equipment, a test is only as good as the samples provided. Timing and handling are especially important for any test measuring microbial activity (such as microbial respiration, nitrogen mineralization, and phospholipid fatty acids, or PLFA). Samples for these tests should be taken during the warm season when plants are actively growing, and ideally should be kept cold until analyzed. While not as critical, soil samples for active organic matter, soil protein/glomalin, and aggregate stability should also be taken during the height of the growing season. For aggregate stability analyses, soil samples should not be dried, ground, or compacted, and need to be kept in bottles or stored in a manner that does not cause the soil to dry out or form clods.
Some commercial soil health test, such as the Cornell and Haney tests, combine several analyses to develop a Soil Health Score.
Every soil health assessment method has its advantages and disadvantages, and soil scientists debate which tests are best for what purposes. For example, soil respiration and nitrogen mineralization are excellent methods for determining how fast soil organisms are decomposing soil organic matter. But these are not good tests for determining soil carbon sequestration. Instead, a measure of soil aggregate stability should be used.
Below, we discuss 13 common soil health assessment methods. This is not meant to be an exhaustive list, and many other methods are certainly possible (just as there are countless ways of assessing human health). Note, for example, that we do not include plant tissue testing, an approach that can provide excellent insights into soil health.
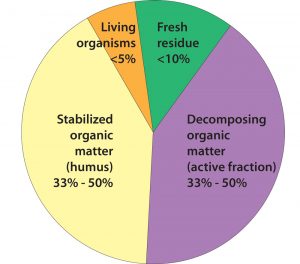
Although it is a very small component of all organic matter, the living component interacts with the dead and very dead components. Source: Ingham, no date
Total soil organic matter tests measure the sum-total of living, dead, and very dead organic matter. Organic matter and the aggregates formed from organic matter have irregular surfaces and are composed of chemical components that bind with soil nutrients such as ammonium, calcium, magnesium, and potassium. This binding ability is called cation exchange capacity (CEC). These binding sites also attach to water molecules, resulting in increased water-holding capacity.
The living and dead forms of organic matter provide food for soil organisms and are involved in organic matter decomposition. Very dead organic matter may be sequestered or trapped inside soil aggregates, bound to soil mineral matter, or (as in the case of lignin) hard for soil organisms to decompose. Nonetheless, it still provides habitat for soil organisms and other benefits. All forms of organic matter combine to help form organic matter coatings on soil mineral particles, enhancing aggregate formation and soil tilth.
While total soil organic matter has served as the standard measurement of soil health for many years, it has several limitations. For one thing, methods of testing soil organic matter differ across laboratories. Each of these methods also has its pros and cons, as illustrated in Table 2.
Another limitation of total soil organic matter testing is that changes in total soil organic matter take place slowly. This is true especially in warm, moist climates favoring high rates of microbial activity and rapid organic matter decomposition, but also in arid climates where plants grow slowly (unless irrigated) or in degraded soils where microbial activity is limited. Assessments of total soil organic matter also do not distinguish between the very different types of organic matter that are found at different depths. Surface soil has primarily living and dead organic matter, while deeper soil has larger amounts of very dead organic matter.
| Soil Carbon Measurement Method | Brief Description | Pros | Cons |
|---|---|---|---|
| Combustion Carbon | High temperature (1300°C) rapid combustion of soil with recovery and detection of CO2 gas. Directly measures total carbon | Rapid; repeatable | Combusts mineral (non-organic) carbon in calcareous soils, leading to overestimation of SOC |
| Loss on Ignition (LOl) | High-temperature (400°C) combustion of soil with difference between weight before and after attributed to SOM | Very inexpensive; repeatable | Certain types of soil (e.g., kaolinites, illites) contain structural water that is lost only at higher than 100°C temperatures, leading to overestimation of SOM |
| Walkley-Black | Chemical oxidation of organic carbon and detection by titrametric color reaction | Avoids errors found in combustion and LOI methods | Hazardous to technicians and uses toxic chemicals; oxidizing potency can be consumed when high clay content or high concentration of certain metals is present, leading to underestimations of SOC |
Total organic matter increases when the amount of organic matter added to the soil is greater than the amount of organic matter decomposed or removed as forage or harvested crop. Growing a high-residue-producing crop, such as sorghum, in rotation with a dense, diverse cover crop, and then using manure or compost for fertility, is a great way to increase organic matter.
Active organic matter testing overcomes a major limitation of total organic matter analysis, since active organic matter levels change far more rapidly, thus providing better feedback on whether management practices are improving soil health. As defined here, active organic matter does not include the very dead component, but only living organisms (earthworms, bugs, beetles, and microscopic organisms), and recently dead plants, animal residues, plant exudates, and animal manure. All of these are considered active since they are readily available to soil organisms involved in organic matter decomposition. The type of organic matter analyzed by active organic matter tests is known as permanganate oxidizable organic matter, due to the chemical (permanganate), used in the test.

A soil sample is reacted with a very caustic reddish-purple chemical, potassium permanganate. The greater the amount of available organic matter, the greater the reaction, causing a decrease in the color of the solution. The amount of color remaining in the solution following the reaction is determined using a spectrophotometer. While research-based analytical assessments of active organic matter require relatively expensive equipment, analyses accurate enough to make farm management decisions can be conducted in the field, using equipment costing less than $1,000.
A limitation of the test is that it does not measure more stable organic matter (the very dead component),which is critical for enhancing CEC, water-holding capacity, and soil carbon sequestration.
You can increase active organic matter by adding easily decomposable forms of organic matter to the soil, such as younger, greener, and softer plant residues, and manure. Older, drier, and more woody materials decompose more slowly and generally contain less active forms of organic matter.
Particulate organic matter tests measure the amount of organic matter that, because of its particle size, is readily decomposable or “labile.”
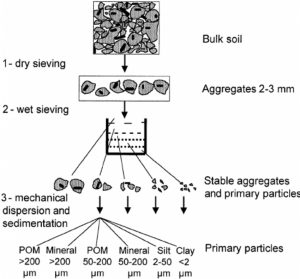
Particulate organic matter is the fraction that is of a specific size. Source: Puget et al., 2008
Soil samples are treated with a chemical that causes aggregates and particles to break apart or disperse. The dispersed soil is sieved and the amount of total organic matter on the sieve is compared to the amount that passes through the sieve to calculate the fraction of organic matter particles that are between 0.053 and 2 mm in diameter. Most particles in this size range are readily decomposable shredded plant and animal material. Assessments of POM are time-consuming, require expensive equipment, and can be difficult to replicate accurately. A simpler, field-based method is available that can be used for preliminary assessments and demonstrations. (See the Further Resources section at the end of this publication for a YouTube video on this method.)
You can increase POM through diverse crop rotations, diverse cover crops, no-till land preparation, and rotational/managed grazing. Studies have shown that POM increases with greater plant biodiversity (Carbandella and Elliott, 1992) and less soil disturbance (Osborne et al., 2014).
Microbial respiration and nitrogen mineralization tests both measure microbial growth or activityand organic matter decomposition, but they measure it in different ways. Microbial respiration testing measures the amount of carbon dioxide (the product of respiration) that is released by soil organisms during a given amount of time for a given amount of soil. In contrast, nitrogen mineralization measures the amount of nitrogen released from the bodies of soil organisms (live organic matter) when they are killed with a toxic chemical.
Researchers can use expensive flux chambers to measure carbon dioxide emissions from soil microorganisms, but the Solvita test is the most common way of measuring soil respiration. A dried and ground soil sample is wetted with water (to stimulate the growth of microorganisms), and placed in a sealed container with a small strip or paddle containing a gel that changes color based on the amount of carbon dioxide produced by microorganisms. (The color can be compared qualitatively with colors on a chart for an approximate value or can be read on a machine for a more accurate value.) This test essentially measures the metabolism rate of decomposer organisms that are breaking down active organic materials and releasing carbon dioxide. Indirectly, these tests also measure aggregate stability and CEC, since respiration rates are increased by reservoirs of nutrients and water stored on stable (very dead) organic matter.
While microbial respiration is easier to measure, nitrogen mineralization is more commonly used in scientific experiments, since it is more precise and less affected by sample-collection and handling methods. Duplicate soil samples are taken. One is exposed to chloroform, killing microorganisms. Next, the ammonium form of nitrogen is extracted from each sample (using potassium chloride) and measured precisely, using chemicals that turn blue based on the amount of ammonium in the sample and a colorimetric analyzer. The difference in ammonium nitrogen levels between the two samples reflects the amount of nitrogen that was released from the bodies of soil organisms killed by chloroform and the amount of nitrogen that could become available to plants during the growing season.
A limitation of all microbial respiration and nitrogen mineralization tests is that microbial growth and activity are strongly affected by soil moisture, temperature, and other conditions. This complicates making comparisons from one date or location to another, or determining the causes of high or low microbial activity. In the laboratory, these tests are conducted at standard temperature and moisture levels, but these conditions vary constantly in the field.
Microbial respiration and nitrogen mineralization are closely related to the amount of active organic matter in the soil. Thus, the same practices that increase active organic matter will also increase microbial respiration and nitrogen mineralization.
The Haney soil test is one of the best-known soil health tests. Three components are combined to yield a Soil Health Score: 1) Solvita test (microbial respiration/activity as determined by CO2 evolution); 2) Water soluble organic C (microbial food); and 3) Water soluble organic N. The Soil Health Score is on a scale of 1 to 100, with a score above seven considered acceptable for many agricultural soils. Native areas, such as grasslands, may achieve a Soil Health Score as high as 100.
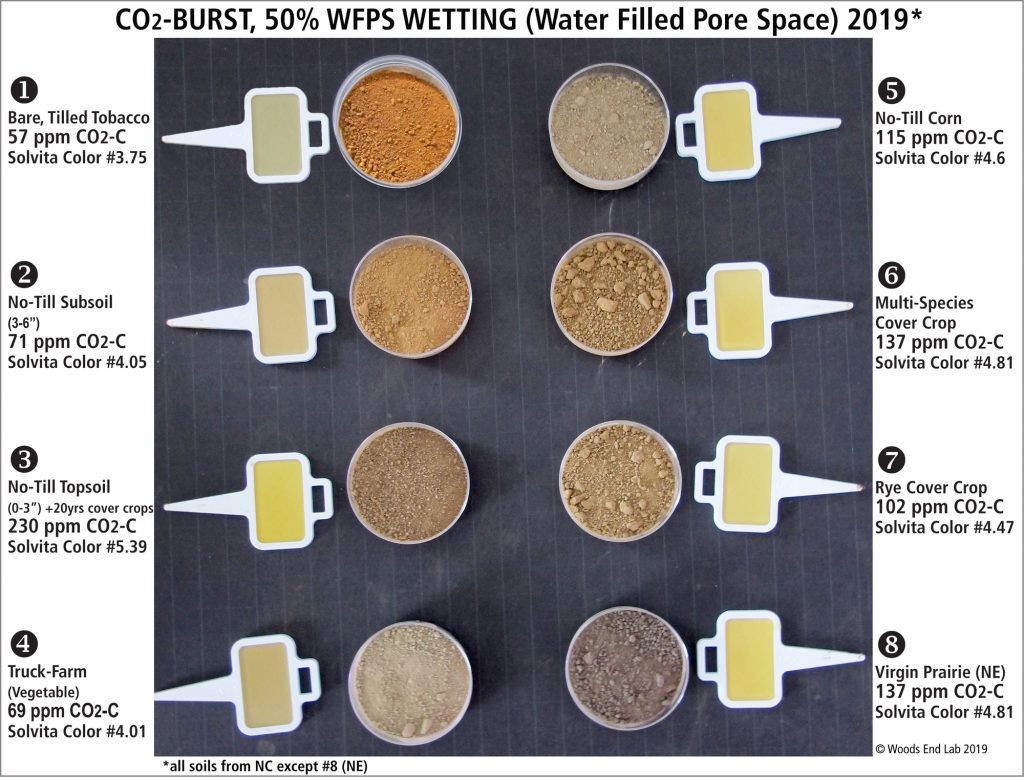
Solvita test, showing color variations. Source: Solvita, no date
The Haney Soil Test combines microbial respiration assessment (using the Solvita test) with assessments of water extractable organic carbon (WEOC) and water extractable organic nitrogen (WEON). WEOC and WEON are both assumed to reflect healthy soil, since WEOC is a carbon source used by soil microbes and WEON is easily broken down by microbes into forms that can be used by plants. The ratio between WEOC and WEON is assumed to be important in microbial activity, with an optimal ratio between 8:1 and 15:1. The Haney Soil Health Score calculation is shown below. In this calculation, 24 hr CO2 -C is the amount of carbon dioxide evolved in the Solvita Test. All variables (24 hr CO2 –C, WEOC, and WEON) are given in parts per million:
Despite the popularity of the Haney test, several research papers have found a limited relationship between Soil Health Score values and agricultural land management practices. (See, for example, Chu et al., 2019; Roper et al., 2017; and Morrow et al., 2016.) Soil sampling and handling methods also affect soil mineralization and respiration values (Roper et al., 2017). One study found that the Solvita component of the test alone had a better correlation with land management practices than the remainder of the test (Yost et al., 2018). Another potential issue is the way the Haney test uses extractable nitrogen values to determine fertilizer or manure application levels. Since solutions used in the Haney test are much more dilute than solutions used in standard soil nutrient analyses, the results from the two tests are not directly comparable.
Since the Haney test primarily assesses mineralizable organic matter, cover cropping, no-till practices, and manure additions may lead to higher Soil Health Scores. As noted above, however, research to date has found a limited relationship between these practices and Haney test results.
Soil protein/glomalin testing assesses mycorrhizal activity, based on a protein that is characteristic to these fungi. Indirectly, this also tests aggregate stability since mycorrhizae are the primary soil organism responsible for forming soil aggregates. Mycorrhizae, as previously discussed, provide multiple benefits to plants, such as increasing their access to phosphorus and water and protecting them against pathogens.
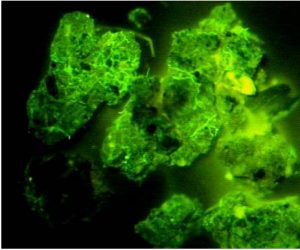
Accumulation of glomalin around mycorrhizal hyphae, as viewed under a microscope. Source: USDA ARS, no date
Soil protein/glomalin testing involves treating soil samples with a dilute salt solution and then autoclaving (a laboratory-based method similar to pressure cooking) the soil for 45 minutes, followed by centrifuging to separate the soil from the soil solution. The amount of protein in the soil solution is analyzed using a chemical that reacts with protein in the solution to form a colored reaction. This test procedure is favored by many soil researchers because it has been shown to be highly responsive to changes in soil management practices.
Since it measures protein found in mycorrhizae and other organisms involved in the formation of soil aggregates, soil protein/glomalin testing provides only indirect evidence about soil properties related to soil decomposition, such as active organic matter levels or microbial activity. While these tests require sophisticated equipment, less expensive equipment (under $1,000) can be used to provide a close approximate value (Wright and Jawson, 2001).
Conditions that favor mycorrhizal growth also favor soil protein/glomalin levels. These conditions include minimal soil disruption, the presence of living plants in the ground throughout the year, and diverse plant species.
Aggregate stability testing measures the resistance of soil aggregates to breakdown or degradation. Aggregates are clumps of soil composed of mineral material held together by mycorrhizae, fine plant roots, earthworm slime, and residues of dead soil organisms. Besides serving as glue to hold aggregates together, these materials serve as food and habitat for soil organisms. Aggregates provide many soil health benefits: Since they are irregularly shaped, they enhance porosity, increasing water infiltration, aeration, and the movement of water and nutrients through soil. Plant root growth is also more abundant in porous soils, allowing for better plant stabilization and greater access to water and nutrients. Chemically, organic matter coatings on aggregates provide abundant cation exchange sites, enhancing nutrient availability to plants and decreasing the potential for nutrient losses due to leaching and runoff. The hydrophilic organic matter coatings on aggregates also enhance soil water absorption and water-holding capacity.
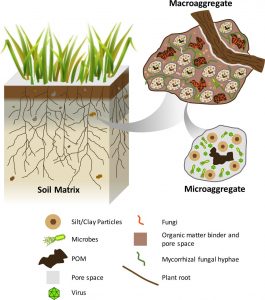
Diagram of a soil aggregate. Source: Jastrow et al., 2007
The oldest and most commonly used method for testing aggregate stability involves placing a soil sample on a nest of soil sieves with screen sizes typically ranging from 1 mm to 45 microns and then moving this nest of sieves up and down in a bucket of water. The more stable aggregates will stay on the top sieve, while less stable aggregates will move through the larger sieves to the finer sieves. The Cornell Soil Health Assessment uses an alternative test where a set of irrigation spigots on horizontal pipes “rain” down on the soil sample, which is on a screen that is placed below the spigots. The percentage of stable aggregates is then calculated based on the weight of soil remaining on the screen in relation to the amount of soil that passes through the screen. Field-based tests involve placing a soil sample on a kitchen sieve that is moved up and down through water.
A simplified “quick and dirty” test of aggregate stability, often used in soil health demonstrations, is the slake test. A handful of soil is placed in a clear jar or beaker, either directly in the water or in a small mesh bag placed in the water. Typically, aggregated soil from a field under conservation tillage is placed into the water in one jar, and non-aggregated soil from a field under conventional tillage is placed in another jar. The aggregated soil typically holds together in the water while the non-aggregated soil turns to mush. See the Further Resources section for a YouTube video that demonstrates this process.
Aggregate stability is widely accepted among soil scientists as a critical indicator of soil health, can be observed in the field without special equipment, and is an excellent indirect indicator of biological activity, water-holding capacity, and the presence of mycorrhizae. Methods for analyzing soil aggregate stability are not consistent across laboratories, however, making precise measurement and comparisons difficult. Good aggregate stability may also have more to do with soil texture than with management practices. For example, soils high in clay usually exhibit a higher aggregate stability than silty or sandy soils, since chemical reactions on the surface of clay minerals allow for effective bonding with organic matter, forming aggregates.
Healthy soil (jar on left) holds together during rainfall. Source: Arriaga, 2014
Aggregate stability is enhanced by all the practices that enhance the growth of mycorrhizae, minimize soil disturbance and compaction, and enhance plant diversity: practices such as reduced- or no-till land preparation and seeding, crop rotations, planting diverse cover crops, and rotational or managed grazing.
PLFAs are biological molecules that are key components of microbial cell membranes. Different types of microorganisms have different types of PLFAs.
The PLFA content of a soil sample is measured with a gas chromatograph, giving an estimate of the size of various microbial communities, such as bacteria and fungi. (Expensive genomic testing would be required to directly analyze and identify the various species of soil microorganisms.) Two components of this analysis—the fungi-to-bacteria ratio and the biomass of mycorrhizae—are particularly associated with enhanced soil health. Both of these characteristics generally increase with organic matter levels, plant diversity, and minimal soil disturbance. A high fungi-to-bacteria ratio also generally reflects higher levels of stable organic matter and plant diversity, since fungi can degrade more complex forms of organic matter than bacteria.
Since PLFAs rapidly degrade following the death of microorganisms, this test only analyzes the living microbial component of soil organic matter.
Environmental conditions can also affect the accuracy of results. Samples must be kept cool until the test is performed. High concentrations of bacteria or more neutral pH levels can result in lower estimates of mycorrhizae than what actually exists (Rousk et al., 2010).
Practices that minimize soil disturbance, ensure a living root in the ground throughout the year, and enhance plant diversity contribute to more favorable PLFA assessments.
Bulk density, water infiltration, and water-holding capacity measure physical characteristics affected by soil organic matter, aggregate stability, and management practices. All are relatively easy to measure in the field on at least a qualitative basis, using low-cost equipment. Compacted soils with a high bulk density restrict root growth, limit movement of water and nutrients to plant roots, and favor anaerobic conditions that slow organic matter decomposition and produce organic acids that inhibit plant growth. Soils with low infiltration rates are susceptible to water runoff and erosion and tend to be drought-prone. Similarly, soils with low water-holding capacity cannot sustain productive plant growth for more than short periods following the onset of a drought.
 |
| Conducting a soil bulk density test. Sources: Soil Quality for Environmental Health, no date; Brown and Wherrett, no date |
Bulk density can be measured in the field with a tool called a penetrometer, which is simply pushed into the ground. Better-quality, digital penetrometers record the density of the soil at 5-centimeter intervals. This information can be used to identify the presence and density of a plow pan, a subsurface clay layer, or soil pores. While relative differences in soil density may remain relatively constant throughout the year, absolute values may differ based on soil moisture. An accurate method of assessing the density of the surface soil involves pounding a sturdy can or a metal ring into the soil, then digging the can out of the soil using a shovel or a trowel, then removing the can from the soil while placing a cover or your hand over the bottom end of the can so none of the soil falls out. The soil in the can is then emptied into an oven-proof dish and completely dried in an oven. Bulk density is the dry weight of the soil divided by the volume of the can.
Water infiltration can be measured roughly and approximately by pouring water onto soil and timing how long it takes to soak into the soil. NRCS recommends using a 6-inch diameter ring, pounded into the ground and lined with plastic wrap. You fill the ring to the one-inch line, then carefully remove the plastic wrap and time how long the water takes to infiltrate. This process is repeated a second time. (The first inch wets the soil, and the second inch gives a better estimate of the infiltration rate.) While not intended for scientific precision, this simple method can be extremely useful, especially for comparing sites or dates or for demonstrations of the impact of enhanced soil organic matter and aggregate stability on water infiltration.
A challenge for water infiltration testing is that measurements are affected by the wetness or dryness of soils at the time of sampling. (Wet soils may absorb water faster or slower than dry soils.) The NRCS ring method attempts to correct for this by wetting the soil first. Researchers overcome this problem, and measure infiltration rates very accurately, by using an expensive ($3,500-$4,000) piece of equipment called a dual-head infiltrometer. This tool saturates the soil and then measures saturated hydraulic conductivity.
Water-holding capacity is the volume of water a given volume of soil can hold. Available water-holding capacity is the portion of this water that’s available to plants: defined as the difference between field capacity and the permanent wilting point. (Field capacity is the moisture level where soils are no longer draining due to gravity, and the permanent wilting point is the moisture level where crops can no longer survive.) There are many ways of measuring or estimating water-holding capacity. Approximate values can be looked up for known soil types and textures. Water can be poured into a known volume of dry soil, noting the amount of water required until the soil begins to drain. A sample of wet soil can be weighed, dried in an oven, and then weighed again to determine the weight and volume of the water held by the wet soil. Some soil labs will also measure and report the water-holding capacity of soil samples sent to them.
Other limitations of bulk density, water infiltration, and water-holding capacity tests:
Reducing or eliminating physical impacts such as using heavy equipment on wet soils and overgrazing will reduce compaction and further degradation in soil structure. But full restoration of these physical properties of soil requires enhancing soil organic matter, aggregate stability, and root and soil organism channels, all of which depend on providing organic matter that feeds and builds soil organism populations.
Plants, residues, and roots promote water infi ltration and reduce runoff comparedto bare soil. Source: USDA NRCS Mississippi, no date
Cation exchange capacity (CEC) and pH are chemical properties of soil that are affected by soil mineralogy, organic matter, and land management practices. Soils with a pH in the neutral range of 6.5 to 7.5 provide beneficial conditions for the growth of most soil organisms involved in soil decomposition, as well as for nitrogen-fixing rhizobia. A high CEC is associated with soil fertility and a soil’s ability to retain nutrients. A moderately high to high CEC suggests that nutrients are available both for plant root growth and the effective decomposition of active soil organic matter. These factors, will, in turn, support mycorrhizal growth, rhizobacteria growth, the presence of active soil organic matter, and aggregate formation.
While soil pH can readily be measured in the field with a handheld pH meter, a sophisticated laboratory analysis—usually an add-on cost to a soil fertility analysis—is necessary to obtain CEC values.
CEC and pH testing only provide partial and indirect evidence about the biological component of soil health. While land management practices can influence CEC and pH values, soil mineralogy and local climate conditions generally have a much greater impact on these soil characteristics. Typically, prairie soils such as those in the U.S. Midwest have CEC values between 60 and 120 meq/100 g soil (a measurement used by soil scientists), while soils in the Southeast U.S. have a type of clay mineral that only provides 10 to 25 meq/100 g soil. Sandy soils and very weathered tropical soils may have even lower CEC values.
Similarly, pH is strongly associated with soil minerology. Soils derived from old sea beds that contain limestone have a relatively high pH (7.5 or higher), whereas highly weathered tropical soils have pH values of 5.5 or less.
Increasing soil organic matter can increase CEC and cause the soil to have a more neutral pH. A relatively small increase in organic matter can cause a large increase in CEC. Application of ammonium fertilizers can cause soil pH to decrease, due to chemical and biological processes that occur in the soil.
Soil texture is a physical property resulting from the interaction between the rock minerals that formed in the soil and the various climate-based, physical, chemical, and biological processes that impacted soil formation over millennia. Soil scientists describe soils as having some combination of three soil textures: sand, silt, and clay. Sand is coarse and gritty, silt feels silky, and clay is sticky.
A soil fertility testing laboratory can determine soil texture, and with practice you can learn to feel a soil’s texture by rubbing a wetted sample of soil between your fingers, noting the amount of grittiness, silkiness, or stickiness.
Soil texture is a purely physical aspect of soil and therefore should not be considered a direct indicator of soil health. Nonetheless, it is mentioned here because it does have an indirect impact on soil aggregate stability, CEC, compaction, bulk density, and water infiltration. For example, clay is composed of very fine particles that easily pack together, resulting in a high potential for compaction and low water-infiltration rates. In contrast, sandy soils are composed of coarse particles that do not pack together well. Sandy soils provide flow paths for water infiltration, but have poor water-holding ability.
Soil texture cannot be changed by soil-management practices, although adding organic matter can increase the formation of soil aggregates, making clayey soil less likely to become compacted and developing greater CEC and water holding capacity in sandy soil.
Healthy soils carry out a wide variety of functions, such as promoting plant growth through organic-matter decomposition, reducing soil loss or contamination through enhanced aggregation, and protecting against drought and climate change through carbon sequestration. Soil health tests typically measure a soil’s capacity to carry out one or two functions, but no test can possibly measure all of them.
Thus, the first question you need to ask in choosing a soil health assessment is, “What characteristic of soil health am I interested in analyzing?” The second and third questions you need to ask are, “How long have soil health practices been used?” and “How do local soil and climate conditions affect the pace of changes in soil health?” Some soil health indicators, such as active organic matter, change relatively rapidly with changes in management practices, while others, such as total organic matter, change very slowly. Initial soil conditions and local climate also influence the time required for changes to become apparent, with tropical and arid conditions taking an especially long time to produce observable changes in soil health.
Table 3 will help you choose an appropriate soil health assessment based on your interest and the pace of the changes you are trying to observe or measure.
| Soil Function Measured | Time Required for Change in Property | Cost per Sample | |||
| Method or Test | Plant Growth | Soil physical & Chemical Properties | Carbon Sequestration | ||
| 1. Soil nutrients | x | R | $15 | ||
| 2. Total soil organic matter | x | x | x | S | $35 |
| 3. Active soil organic matter | x | R | $20 | ||
| 4. Microbial respiration | x | x | M | No price, primarily used in research | |
| 5. N mineralization | x | x | R | $15-$25 | |
| 6. Soil protein/glomalin | x | R | No price, primarily used in research | ||
| 7. Particulate Organic Matter (POM) | x | x | x | R | $50 |
| 8. Phospholipid Fatty Acid (PLFA) | x | x | M | $20-$30 | |
| 9. Haney soil health test | x | x | M | $30-$55 | |
| 10. Aggregate stability | x | M | $50-$80 | ||
| 11. Bulk density | x | M | $10 | ||
| 12. Water infiltration | x | x | M | No price, in field assessment | |
| 13. Water-holding capacity | x | x | M | $25 | |
| 14. Soil texture | x | M | $10 | ||
| 15. Cation Exchange Capacity (CEC) | x | R | Comes with test #1 | ||
| 16. pH | x | NA | $35 | ||
| 17. Cornell Soil health test (includes 1, 2, 3, 4, 6, 9, 13, 15 | x | x | x | R | $110 |
| R=Rapid (1- 2 years), M=Medium (2-5 years), S=Slow (>5 years). Times are approximate and affected by soil, climate, and management. | |||||
Arriaga, F. 2014. Soil Aggregation and Water Infiltration. University of Wisconsin Integrated Pest and Crop Management.
Brown, K. and A. Wherrett. No date. Bulk Density – Measurement. Fact sheet.
Cambardella, C., and E. Elliott. 1992. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Science Society of America Journal. Vol. 56. p. 777-783.
Chu, Mingwei, S. Singh, F.R. Walker, N.S. Eash, M.J. Buschermohle, L.A. Duncan, and S. Jagadamma. 2019. Soil health and soil fertility assessment by the Haney soil health test in an agricultural soil in West Tennessee. Communications in Soil Science and Plant Analysis.
Vol. 50, No. 9. p. 1123-1131.
Conservation Media Library. 2016. Prairie Strips. Flickr.com.
Doran, John W., and Michael R. Zeiss. 2000. Soil health and sustainability: managing the biotic component of soil quality. Applied Soil Ecology. Vol. 15, No. 1. p. 3-11.
Haney, Richard L., E.B. Haney, D.R. Smith, R.D. Harmel, and M.J. White. 2018. The soil health tool—Theory and initial broad-scale application. Applied Soil Ecology. Vol. 125. p. 162-168.
Haney, R.L, W.F. Brinton, and E. Evans. 2008. Soil CO₂ respiration: Comparison of chemical titration, CO₂ IRGA analysis and the Solvita gel system. Renewable Agriculture and Food Systems. Vol. 23, No. 2. p. 171-176.
Hoorman, James J., and R. Islam. 2010. Understanding Soil Microbes and Nutrient Recycling. Ohio State University Extension, Columbus, OH.
Ingham, Elaine. No date. Soil Food Web. USDA Natural Resources Conservation Service. Jastrow, J.D., J.E. Amonette, and V.L. Bailey. 2007. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Climatic Change. Vol. 80. p. 5-23.
Kumar, Ashok, A. Arokia Prakash, and Bhavdish Narain Johri. 2011. Chapter 2: Bacillus as PGPR in Crop Ecosystem. In: Maheshwari, Dinesh (ed.). Bacteria in Agrobiology: Crop Ecosystems. Springer-Verlag Berlin Heidelberg. p. 37-59.
Moore, Lisa Schulte. 2014. Prairie Strips: Bringing biodiversity,
improved water quality, and soil protection to agriculture. Missouri Prairie Journal. Vol. 35, No. 1. p. 12-15.
Morrow, Jason G., D.R. Huggins, L.A. Carpenter-Boggs, and J.P. Reganold. 2016. Evaluating measures to assess soil health in long‐term agroecosystem trials. Soil Science Society of America. Vol. 80, No. 2. p. 450-462.
NRCS. No date. Soil Health. USDA Natural Resources Conservation Service.
Osborne, S.L., J.M.F Johnson, V.L. Jin, A.L. Hammerbeck, G.E. Varvel, and T.E. Schumacher. 2014. The impact of corn residue removal on soil aggregates and particulate organic matter. BioEnergy Research. Vol. 7. p. 559–567.
Puget, P., C. Chenu, and J. Balesdent. 2008. Dynamics of soil organic matter associated with particle‐size fractions of water‐stable aggregates. European Journal of Soil Science. Vol. 51. p. 595- 605.
Roper, Wayne R., D.L. Osmond, J.L. Heitman, M.G. Wagger, S.C. Reberg-Horton. 2017. Soil Health Indicators Do Not Differentiate among Agronomic Management Systems in North Carolina Soils. Soil Science Society of America. Vol. 81, No. 6. p. 828-843.
Rousk, Johannes, P.C. Brookes, E. Bååth. 2010. The microbial PLFA composition as affected by pH in an arable soil. Soil Biology & Biochemistry. Vol. 42, No. 3. p. 516-520.
Soil Quality for Environmental Health. No date. Bulk Density
Todd, Chris. No date. Mycorrhizal Fungi, Nature’s Key to Plant Survival and Success. Pacific Horticulture Society.
Ward Laboratories. 2018. Haney Test.
Wright, S.F. and L. Jawson. 2001. A pressure cooker method to extract glomalin from soils. Soil Science Society of America Journal. Vol. 65, No. 6. pp. 1734-1735.
Yost, M.A., K.S. Veum, N.R. Kitchen, J.E. Sawyer, J.J. Camberato, P.R. Carter, R.B. Ferguson, F.G. Fernández, D.W. Franzen, C.A. Laboski, and E.D. Nafziger. 2018. Evaluation of the Haney Soil Health Tool for corn nitrogen recommendations across eight Midwest states. Journal of Soil and Water Conservation. September. Vol. 73, No. 5. p. 587-592.
Procedures for 12 on-farm tests.
A short video demonstration of the soil stability test.
Shows a streamlined method, which takes 20 minutes or less, for assessing particulate organic matter in soils.
Soil health tests includes respiration, extractable organic
carbon, extractable organic nitrogen, mineralizable carbon, total nitrogen, organic C:N.
Soil health tests include soil pH, organic matter, wet aggregate stability, soil respiration, soil protein.
Soil health tests include Solvita 24 hour CO2 Burst Test (respiration), Haney soil test.
Soil health tests include Haney soil health, organic matter, and Solvita respiration.
Soil health tests include PLFA, Haney soil test, soil respiration, enzymes, active organic matter, wet aggregate stability, and available water-holding capacity.
Soil health tests include Solvita 24-hour CO2 Burst Test
(respiration), VAST (aggregate stability)
An overview of soil health characteristics and management practices.
An interactive web-based tool to provide information on farm management practices that enhance soil health.
Building Soils for Better Crops. 3rd Edition. By Fred Magdoff and Harold van Es. SARE. College Park, MD.
An online version of one of the earliest books to provide an easy-to-read yet comprehensive and scientific description of soil health characteristics and the impact of farm management practices on enhancing soil health.
A private-public partnership conducting research, providing education, and influencing policies on soil health. The website provides links to videos, technical articles and a comprehensive list of magazines, research studies, blogs, and websites that address issues of soil health.
A portal to NRCS soil health resources, such as soil quality indicator sheets and a soil health assessment manual.
Soil Health Indicators and Tests
By Barbara Bellows, Texas Institute for Applied Environmental Research; Mike Morris, NCAT; and Colin Mitchell, NCAT
Published November 2020
© NCAT
IP603
Slot 629
This publication is produced by the National Center for Appropriate Technology through the ATTRA Sustainable Agriculture program, under a cooperative agreement with USDA Rural Development. ATTRA.NCAT.ORG.